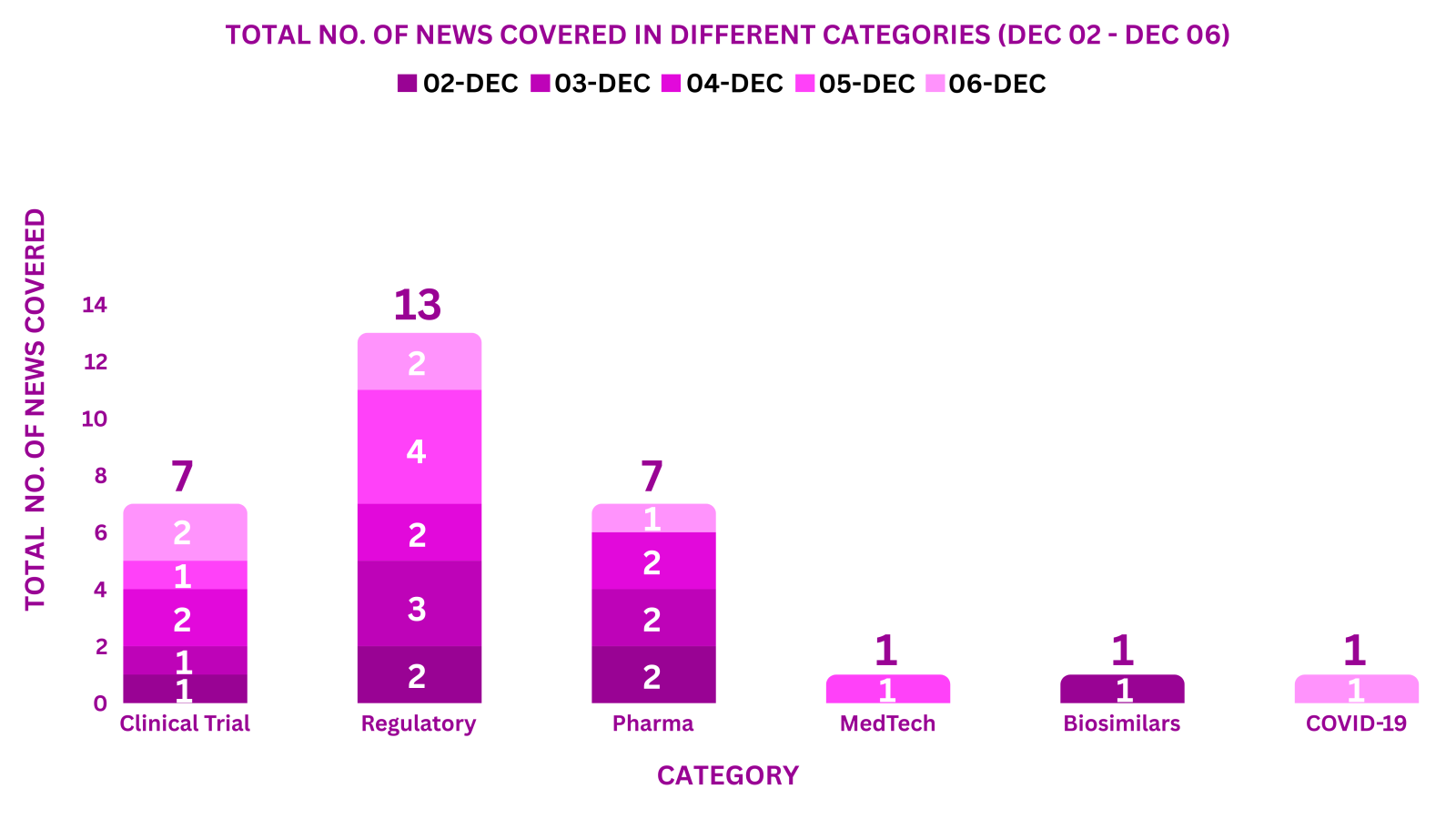
PharmaShots Weekly Snapshots (December 02 – December 06, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, Biosimilars & COVID-19. Check out our full report below:

Minghui Pharmaceutical Reports Topline Data from P-III Study of Tofacitinib Etocomil (MH004) Ointment for Atopic Dermatitis
Read More: Minghui Pharmaceutical
InventisBio Reports Results from the P-II Trial of D-2570 to Treat Moderate to Severe Plaque Psoriasis
Read More: InventisBio
Everest Medicines Reports Results from the P-Ib/IIa Study of EVER001 to Treat Primary Membranous Nephropathy
Read More: Everest Medicines
Eli Lilly Reports Results from the P-IIIb (SURMOUNT-5) Trial of Zepbound (Tirzepatide) in Obese Adults
Read More: Eli Lilly
VivaVision Biotech Reports Topline Results from P-II Study of VVN461 for Post-Operative Inflammation Following Cataract Surgery
Read More: VivaVision Biotech
Daiichi Sankyo Reports Pooled Analysis from TROPION-Lung05 & TROPION-Lung01 Trials of Datopotamab Deruxtecan (Dato-DXd) for NSCLC
Read More: Daiichi Sankyo
Duality Biologics and BioNTech Highlight Interim Data from P-I/IIa Trial of BNT324/DB-1311 in Advanced Solid Tumors at ESMO Asia 2024
Read More: Duality Biologics and BioNTech

Renalys Pharma’s Sparsentan Secures the MHLW’s Orphan Drug Designation to Treat Primary IgA Nephropathy
Read More: Renalys Pharma
Revelation Biosciences Reports the US FDA’s IND Acceptance of Gemini for Chronic Kidney Disease
Read More: Revelation Biosciences
Cytokinetics Reports the US FDA’s NDA Acceptance of Aficamten to Treat Obstructive Hypertrophic Cardiomyopathy
Read More: Cytokinetics
Rigel’s R289 Secures the US FDA’s Fast Track Designation for Treating Lower-Risk Myelodysplastic Syndrome
Read More: Rigel
HUTCHMED and Innovent Report the NMPA’s Conditional Approval of Elunate Plus Tyvyt to Treat Advanced Endometrial Cancer
Read More: HUTCHMED and Innovent
Merck’s Sacituzumab Tirumotecan (Sac-TMT) Secures the US FDA’s Breakthrough Therapy Designation to Treat Non-Squamous NSCLC
Read More: Merck
Allay Therapeutics’ ATX101 Secures the US FDA’s Breakthrough Therapy Designation to Treat Post-Surgical Pain
Read More: Allay Therapeutics
Merus Reports the US FDA’s Accelerated Approval of Bizengri (Zenocutuzumab-zbco) for NRG1+ Pancreatic Adenocarcinoma and NSCLC
Read More: Merus
Roche Reports the US FDA’s sBLA Acceptance of Columvi (Glofitamab) Combination to Treat R/R Diffuse Large B-Cell Lymphoma (DLBCL)
Read More: Roche
AstraZeneca’s Imfinzi (Durvalumab) Secures the US FDA’s Approval for Treating Limited-Stage Small Cell Lung Cancer
Read More: AstraZeneca
Phanes Therapeutics’ PT217 Secures the US FDA’s Fast Track Designation for Neuroendocrine Prostate Cancer (NEPC)
Read More: Phanes Therapeutics
AstraZeneca Reports the US FDA’s sBLA Acceptance and Priority Review of Imfinzi to Treat Muscle-Invasive Bladder Cancer
Read More: AstraZeneca
Affimed Secures the US FDA’s Regenerative Medicine Advanced Therapy (RMAT) Designation for Acimtamig and AlloNK Combination
Read More: Affimed

Helix Biopharma to Acquire Laevoroc Immunology’s LR 09, Expanding its Immune-Oncology Portfolio
Read More: Helix Biopharma and Laevoroc Immunology
Silo Pharma and Kymanox Join Forces for SP-26 Ketamine Implant Device Targeting Pain Management
Read More: Silo Pharma and Kymanox
Merus Collaborates with Partner Therapeutics to Commercialize Zenocutuzumab for Treating NRG1 Fusion-Positive Cancer
Read More: Merus and Partner Therapeutics
PTC Therapeutics Join Forces with Novartis to Develop PTC518 for Treating Huntington's Disease
Read More: PTC Therapeutics and Novartis
Lisata Therapeutics Collaborates with Kuva Labs to Advance Non-Invasive, High-Precision Cancer Diagnostics
Read More: Lisata Therapeutics and Kuva Labs
Relay Therapeutics and Elevar Therapeutics Collaborate to Develop Lirafugratinib (RLY-4008) for Cholangiocarcinoma and Other Solid Tumors
Read More: Relay Therapeutics and Elevar Therapeutics
Muna Therapeutics Partners with GSK to Develop Novel Treatments for Alzheimer’s Disease
Read More: Muna Therapeutics and GSK

Carlsmed Reports the US FDA’s Approval of aprevo Cervical Breakthrough Fusion Device
Read More: Carlsmed

Biocon Biologics Reports the US FDA’s Approval of Yesintek (Biosimilar, Stelara)
Read More: Biocon Biologics

Merck and Ridgeback Biotherapeutics Begin P-III (MOVe-NOW) Trial of Lagevrio (Molnupiravir) to Treat COVID-19 in High-Risk Adults
Read More: Merck and Ridgeback Biotherapeutics
Related Post: PharmaShots Weekly Snapshots (November 25 – November 29, 2024)
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.